Alcohol Miscible in Water
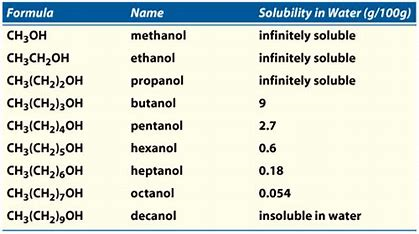
35 rows The following compounds are liquid at room temperature and are completely. Solubility of alcohols is therefore determined by the stronger of the two forces.
Why Are Low Molecular Weight Alcohols Soluble In Water And Why Does The Water Solubility Of Alcohols Decrease With The Increasing Size Of The Hydrocarbon Group Can You Explain This Socratic
Therefore ethyl alcohol is miscible with water.

. Thus it is completely miscible. Why is ethanol miscible in water. Isopropyl alcohol boils at 826 0 C while the water.
The hydroxyl group is referred to as a hydrophilic water-loving group because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water. -- The above answer is correct if asking if each solvent is miscible in water. It is the unique angle between two hydrogen and.
Because of the strength of the attraction of the OH group first three alcohols methanol ethanol and propanol are completely miscible. The hydroxyl group in alcohol is known as hydrophilic water-loving which forms hydrogen bonds with water making it soluble. Â When mixed with.
Solubility of alcohols is therefore determined by the stronger of the two forces. Ethanol does on a molecule by molecule basis have stronger intermolecular forces between itself and water than methanol and water. CAS 67-63-0 EINECS 200-661-7 Azer Scientific Catalog Nos ES602.
Methanol ethanol n-propyl alcohol isopropyl alcohol and t-butyl alcohol are all miscible with water. Water is also one type of alcohol if you consider the formula H-OH. The general formula of alcohol is R-OH.
Ethanol is a type of alcohol that is considered miscible or soluble in water. Thus it is completely miscible. Ethyl alcohol is immiscible with.
Hydrogen bonds are formed in many compounds eg H 2OHFNH 3. Distillation Distillation Process Isopropyl Alcohol With Salt Water Checking Miscibility Share. Isopropyl alcohol is a clear colourlesss solvent and is miscible in water and hydrocarbon solvents.
The boiling point of such compounds depends to a large extent on the strength of hydrogen bonds. In hydroxyl group of alcohol the electronegativity values of oxygen and hydrogen are 35 21 respectively ie the. Ethyl alcohol and water dissolve in each other in all proportions.
Alcohols with higher molecular weights tend to be less water. Chemistry questions and answers. Salting Out Effect Of Water And Methyl Alcohol Methylation Alcohol Salt.
Methanol is miscible in water but Ethyl Acetate is immiscible in water. September 3 2012 erwin Leave a comment. Answer 1 of 6.
Ethanol is a type of alcohol that is considered miscible or soluble in. Isopropyl alcohol is miscible in water ethanol and chloroform as like these compounds isopropyl is a polar moleculeIt dissolves ethyl cellulose polyvinyl butyral many oils. Bond dipole moment is the measure of the polarity of a chemical bond.
Which alcohol is miscible with water. Molecular weight - Alcohols with lower molecular weights like. A liquid with a similar polarity to water should therefore be miscible in water.
N-amyl alcohol also called 1-pentanol. If you are asking if they. It is measured in 0 to.
Yes water is miscible in methyl alcohol which is also known as methanol. Starting with the four-carbon butanol the solubility of alcohols is starting to decrease. Immiscible means that they cannot be mixed into a solution- ie water and oil Are.
And more soluble in water than. Another thing that happens when you mix you isopropyl alcohol and water together is a solution with a boiling point of 8037 0 C. Miscible means that two liquids can be mixed in any proportion- ie water and alcohol.
They dissolve in water in any amount. While methanol has formula CH3-OH and ethanol C2H5-OH. Alcohol and water form a miscible solution because alcohol is soluble in water and oil are not soluble in water hence it forms an immiscible solution.
Is Alcohol Soluble In Water Techiescientist

2 Properties Of Alcohols Alcohol Carboxylic Acid And Esters

No comments for "Alcohol Miscible in Water"
Post a Comment